Trach Sense
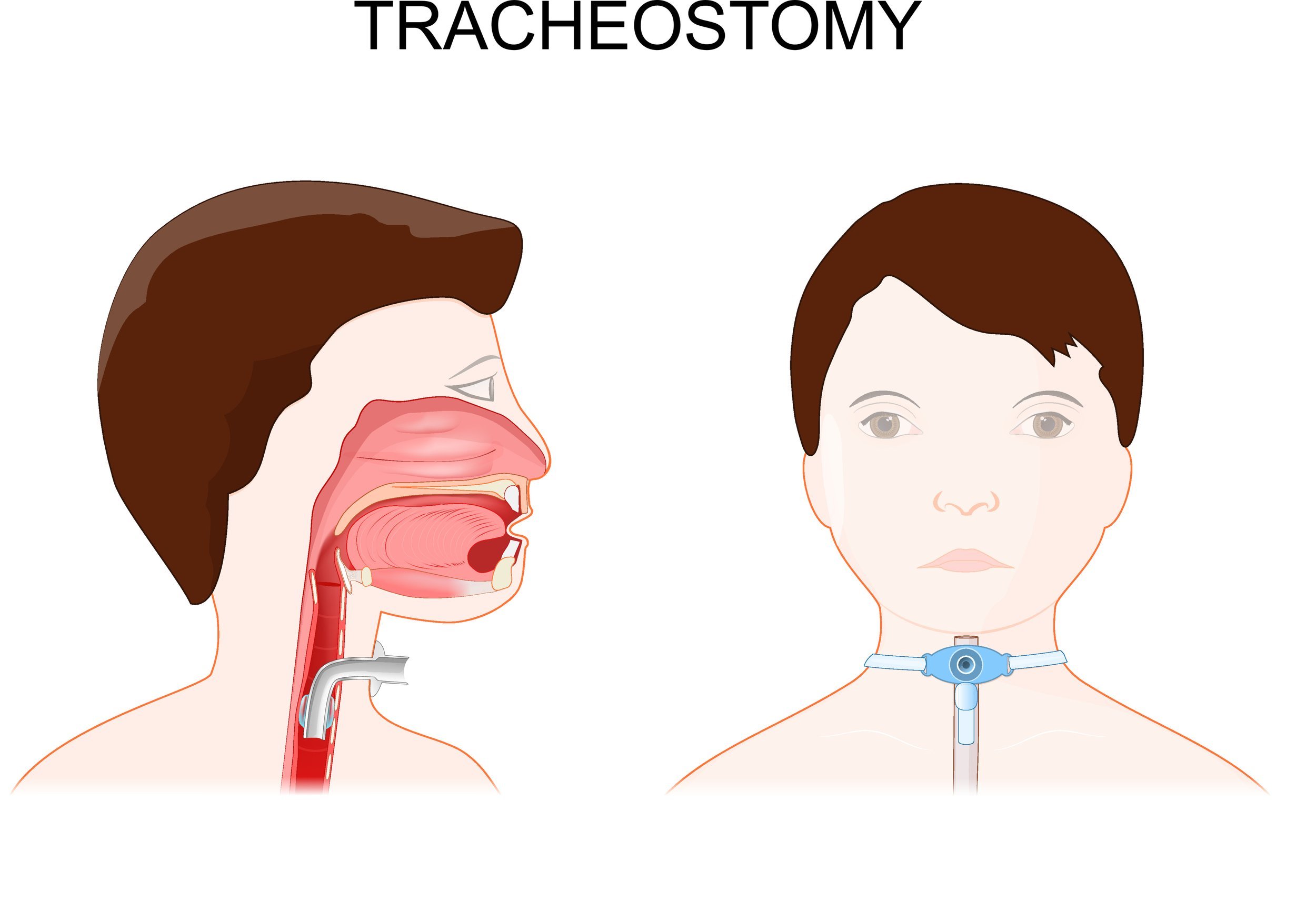
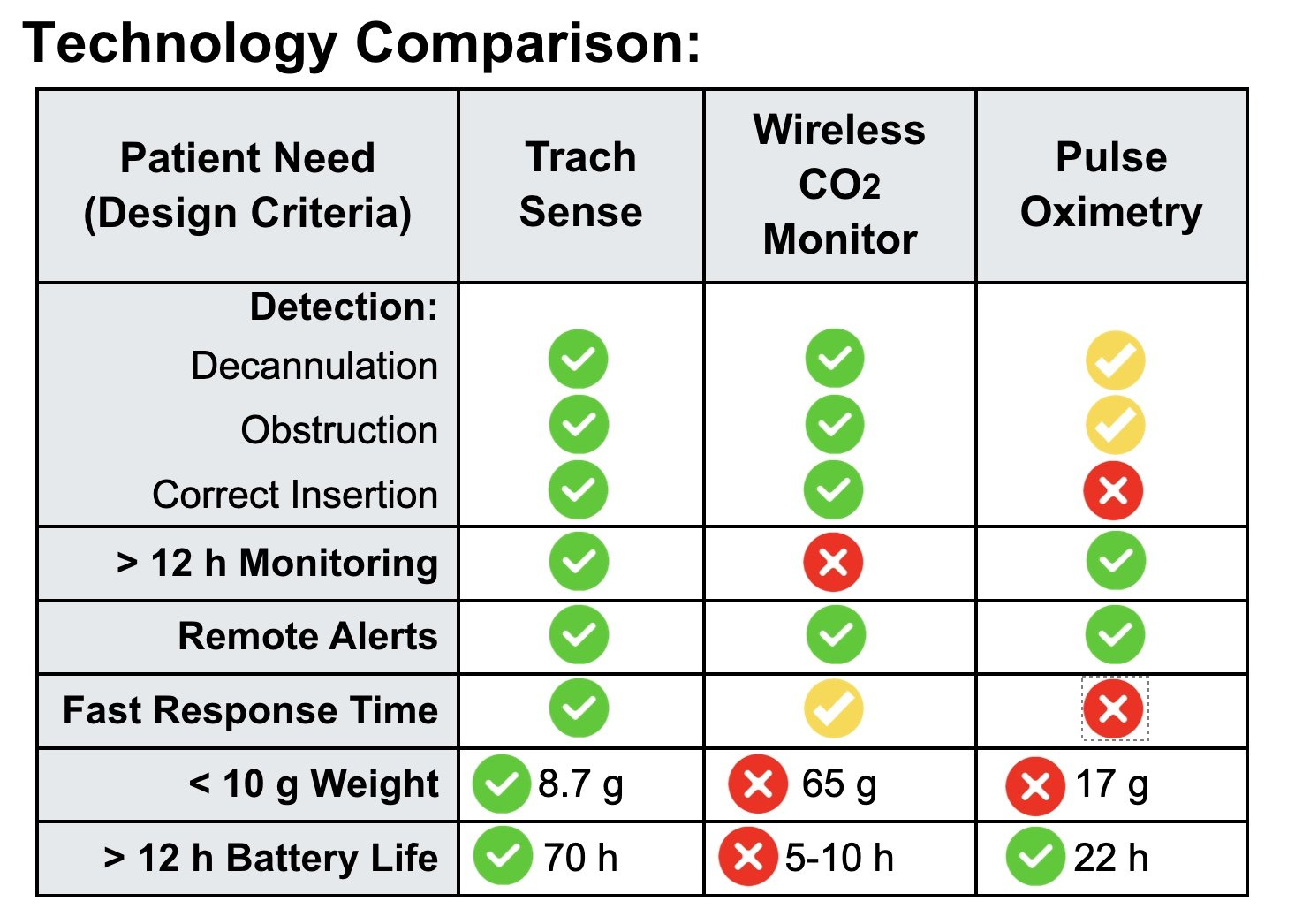
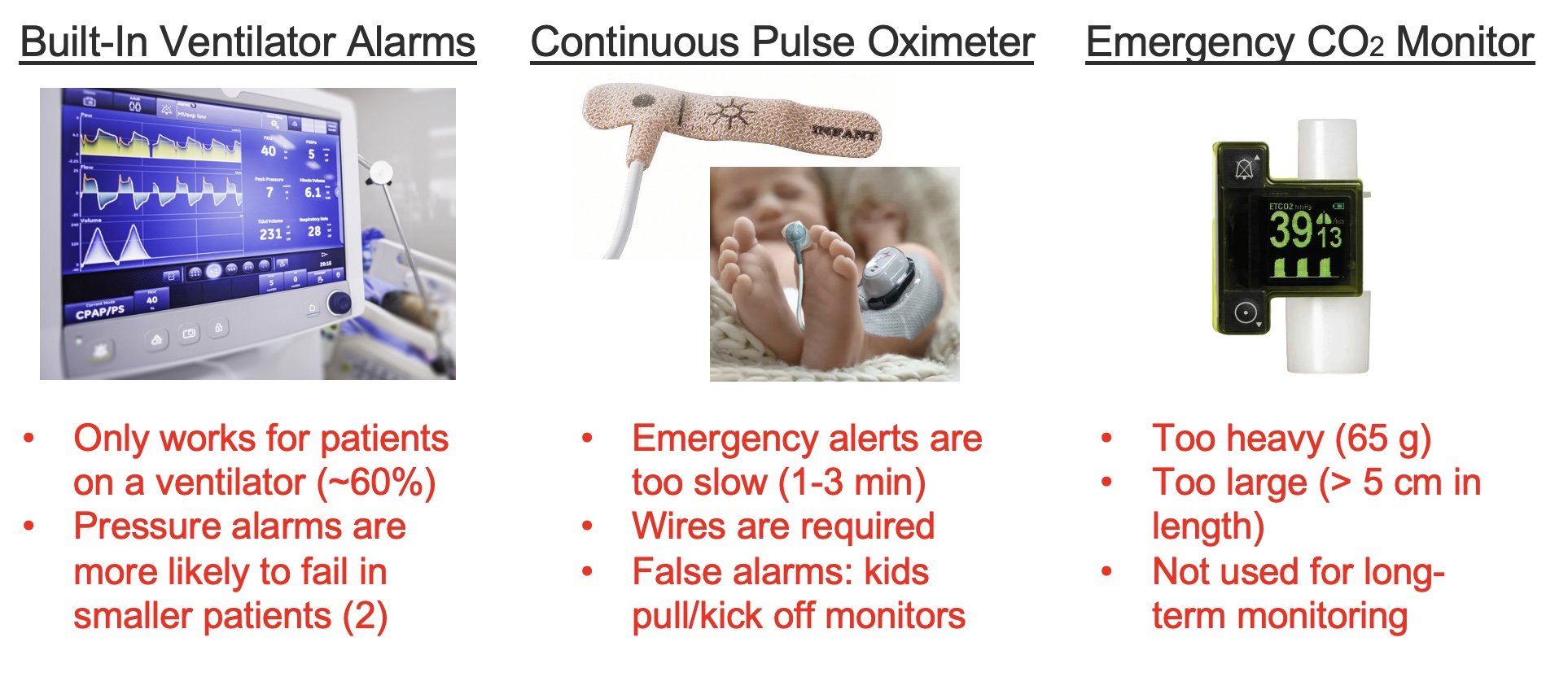
Problem: Emergencies involving tracheostomies, such as improper placement, obstruction, and accidental decannulation, pose critical risks, particularly in pediatric patients. These events can result in severe injury or death within minutes due to delayed detection. Current monitoring technologies, such as pulse oximeters, fail to provide real-time alerts for tracheostomy-specific complications.
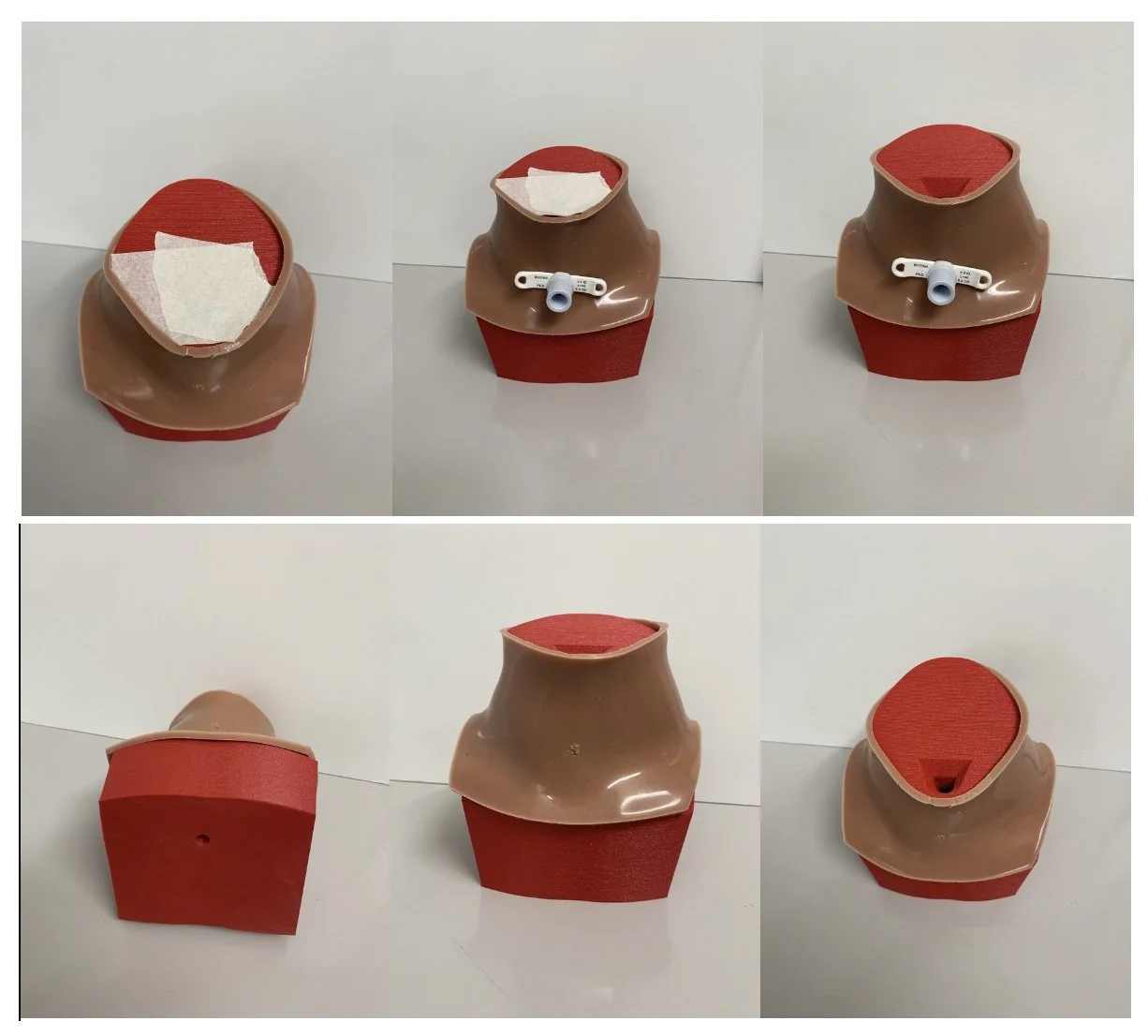
Solution: Trach Sense is a compact, lightweight CO2 monitoring device that detects airway emergencies within 20 seconds, integrates seamlessly with standard tracheostomy tubes, and provides real-time alerts via a mobile app.
Children’s National Hospital | Washington, D.C. | APDI and CNH Funded | 2024
The Trach Sense device is designed to address critical tracheostomy tube complications, such as accidental decannulation and obstructions, which are common in pediatric patients who are mobile and not ventilator-dependent during day-time hours. Complications can lead to severe outcomes, including neurological damage or death if not identified promptly. Trach Sense is a compact, wireless attachment that continuously monitors CO2 levels in real-time, providing rapid alerts to caregivers via a mobile app within 20 seconds of detecting an issue. This innovative solution improves response times, potentially saving lives and reducing hospital readmissions by providing a more reliable alternative to current monitoring methods like visual inspection and pulse oximetry. The device is universally compatible with various tracheostomy tubes, making it suitable for both home and clinical settings, thus supporting caregivers with diverse levels of expertise in managing emergencies. The primary aim is to enhance safety and reduce morbidity amongst pediatric tracheostomy patients. 20% of tracheostomized children experience emergencies like obstruction or decannulation, with a 4–6% mortality rate. Approximately 4,000 pediatric tracheostomies are performed annually in the U.S., with 25,000 globally. Length of time a pediatric patient has a tracheostomy can range from 6 months to a lifetime depending on their physiology and co-morbidities.
Over the past year the Biodesign Program at Children’s National Hospital has been awarded non-diluted funding for developing Trach Sense through Childrens’s Hospital Founder’s Auxiliary Board and The Alliance for Pediatric Device Innovation (APDI). We have filed a patent, engaged early with the FDA through a PCI meeting, and have submitted our FDA Pre-Submission application. We are currently conducting our second phase of pre-clinical testing and planning a clinical pilot study for early 2026. If you are interested in this technology please visit The CNH Innovation Ventures Licensing Office.
Design Team: Jules Sherman, MFA, Kaylee Meyers, Ph.D., Ghee Ong, Ph.D., Ethan L Cooper, Arsalan Siddiqui, Noah Jagdman, Shahmeel Naseem, Tuan Ishaque Aqeel Muthaliff
Patent Pending: Tracheostomy Tube Complication Monitoring Accessory and Uses Thereof